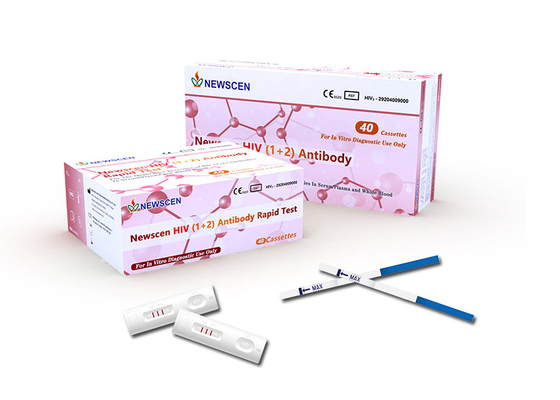
HIV(1+2) Antibody Rapid Test Kit
For qualitative detection of HIV(1+2) antibodies in serum/plasma and whole blood
Main Features
► Sensitivity: 100%
► Specificity: Higher than 99%
► Simple: No Instrument Required
► Ambient Storage
► Reliable: able to differentiate HIV Type I and Type II
► Certified by Authoritative Certification
► Unique 3-line Patented Design
► Winner of "the 2008 National HIV Antigen Diagnostic Kit for Clinical Performance Assessment"

Intended Use
Newscen HIV(1+2) Antibody Rapid Test is a single use, rapid immunoassay for qualitative detection of antibodies to Human Immunodeficiency Virus type 1 and 2 (HIV-1/2) in human serum, plasma or whole blood collected from vein or fingertip. It is intended for use in medical institutions by trained staff.
Principle
HIV-1(gp41 and gp120) and HlV-2(gp36) specific recombinant antigens are separately precoated on to the membrane inzone1and2as the capture reagent on the test zone. During the test, specimen is allowed to react with the colloidal gold particles, which have been conjugated with HIV-1andHIV-2 specific recombinant antigens. Antibodies to HIV-1 and/or HIV-2, if present, will specifically bind to colloidal gold-antigen complex. When the colloidal gold-antigen-antibody complexes move to the test zone, they will specifically bind to the precoated antigens. At the same time, a red colored line will develop in zone 1 and/or 2 on the membrane. Absence of these red colored lines in the test zone ( 1 and 2) suggests a negative result. To serve as a procedural control, red colored line in the control zone will always appear regardless of the presence of antibodies to HIV-1/HIV-2.

Reagents and Materials Provided
Each kit contains:
► 40 test cassettes(individually pouched)
► Each pouch contains one cassette with one desiccant bag
► One bottle of diluent buffer(5ml)
► 40 disposable plastic droppers
► Instruction for use
Materials Required But Not Provided
► Timer or stopwatch
► Blood collection devices, for the testing of venous whole blood, serum or plasma
► Biohazard disposal container
► Disposable gloves
For finger stick samples, the following materials are required:
► Alcohol pad
► Sterile lancet
► Sterile gauze or cotton

Sample Collection and Test Preparation
Finger stick Specimens(Whole Blood)
► Clean the area to be lanced with an alcohol pad.
► Squeeze the end of the fingertip and pierce it with a sterile lancet.
► Wipe away the first drop of blood with sterile gauze or cotton; collect the sample from the second drop.
► Use dropper to obtain appropriate amount of fresh blood and dispense into the sample well.
Finger stick whole blood should be used immediately after collection.
Serum/Plasma specimens:fresh serum or plasma specimen can be used. No special patient preparation required.
Plasma
► Collect whole blood into a collection tube(containing EDTA, Na-citrate or heparin) by venipuncture.
► Separate the plasma by centrifugation.
Serum
► Collect whole blood into a collection tube(containing no anticoagulants) by venipuncture.
► Allow the blood to clot.
► Separate the serum by centrifugation.
Any visible particulate matter in the specimen should be removed by centrifugation or filtration. Avoid using of hemolytic, turbid, microorganism contaminated specimens or specimens stored for over 5 days at 4°C.Specimen should be stored frozen at-20°C for maximum 3 months. Avoid specimen deterioration by multiple freeze-thaw cycles.
Venous Whole Bloods
Venous whole blood can be used immediately after collection or stored up to 4 days at 2-8°C.

Assay Procedure
1.Place the test cassette on flat surface. Before unseal the pouch, allow the test cassette to reach room temperature(4-30°C) .Use it immediately once unsealed.
2.Open the pouch and add1drop(30-40uL) of specimen into the sample well(S) .
3.When the specimen is completely absorbed, slowly add1drop(45-55pL) of diluent buffer vertically into the sample well(s) .
4.Avoid dropping specimen or diluent buffer in the observation window.
5.Do not allow the diluent buffer bottle touch the sample well when dropping the diluent buffer so as to prevent the cross contamination with the specimen.
6.Observe the result between 15-30 minutes after the diluent buffer added.
Interpretation of Results

Note:‘C'-Control line; ‘1'-HIV-1 Test line; ‘2'-HIV-2 Test line
Negative: No redlines appear within 30 minutes in the test zone( 1 and 2), only a redline in the control zone(C) , which indicates that no antibodies to HIV 1+ 2 have been detected with this test. However, this does not exclude the possibility from infection with HIV.
Positive: One redline in the control zone(C) and one or two red visible lines of any intensity in the test zone( 1 and 2) .This indicates the specimen contains HIV-1 and/or HIV-2 antibodies.
Invalid:No redlines appear in the control zone(C), regardless of whether there is a redline in the test zone( 1 and 2) , indicating that the test is invalid. Discard the test cassette and perform with new cassette.
Built-In Control
Newscen HIV(1+2) Antibody Rapid Test has a built-in procedural control that demonstrates assay validity. A redline appeared on the control zone(C) indicates that the test runs correctly.
Storage
Newscen HIV(1+2) Antibody Rapid Test should be stored at room temperature(4-30℃, do not freeze)
for 24 months from the date of manufacture. Keep the test cassette in sealed pouch until use. Once you have taken the test cassette out of the pouch, perform the test as early as possible(within 1hour) to avoid test cassette from becoming moist. Do not use the test beyond the indicated expiration date.
The diluent buffer should be stored at room temperature(4-30℃, do not freeze) .
Warning
For Invitro Diagnostic Use ONLY
Read the package insert completely before use. It is very important that the correct procedure is followed. Fail to add the patient sample may lead to a false negative result (i.e.a missed positive) .



 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!