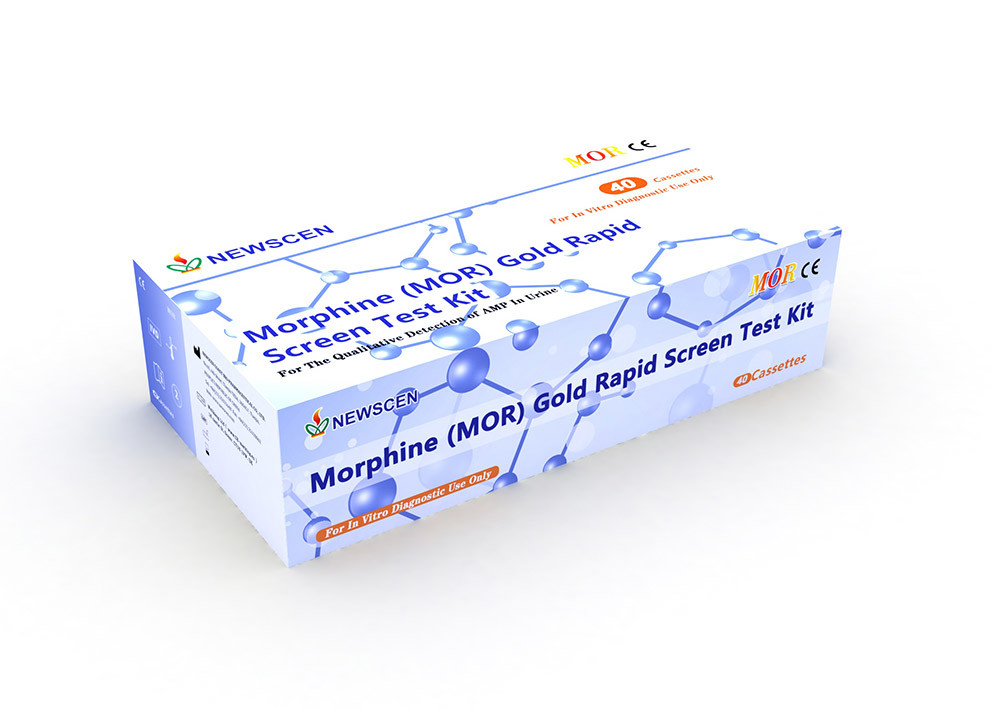
MOR (Morphine) Gold Rapid Screen Test Cassette
For the Qualitative Detection of MOR in urine
Home Use Qualitative Detection Of MOR In Urine Morphine Rapid Test Kit For Drug Abuse
Main Features
☀ High Sensitivity
☀ High Specificity
☀ Reliable: high accurate, early detection of the presence of MOR
☀ Simple: No Instrument Required
☀ Convenient: Room Temperature Storage, Built-In Control line
☀ Fast: Results in 5-30 minutes, strong positive results may be observed promptly within 3 minutes
☀ Certified by Authoritative Certification System and Standards
☀ Winner of "the National Rapid Diagnostic Kit for Clinical Performance Assessment"
INTENDED USE
The MOR Gold Rapid Screen Test Cassette is a rapid qualitative competitive binding immunoassay for determination of Morphine and their metabolites in urine.

SUMMARY AND EXPLANATION OF THE TEST
The MOR Gold Rapid Screen Test Cassette is a fast and easy to read membrane chromatographic immunoassay with no need for instrument. The method employs a unique combination of monoclonal and polyclonal antibodies to specifically identify Morphine in urine with a high degree of sensitivity.
Morphine, one of opiates’ derivatives, is central nervous system stimulants that produce alertness, wakefulness, increased energy, reduced hunger, and an overall feeling of well-being.
Morphine has been the preferred drug for the management of pain in cases of advanced cancer and other severe conditions. Morphine and other Opiates’ metabolites may be detected in urine as a result of heroin morphine, codeine, or poppy seed intake. Administration of large doses of Morphine can cause tolerance and physiological dependence in users, and can lead to substance abuse. D-Morphine is controlled substances. GC/MS or immunoassay method has been developed for the determination of 300ng/ml for Morphine.

REAGENTS AND MATERIALS PROVIDED
1. Test Device
A pouched cassette contains a single test for Amphetamine
2. Dropper
A transfer pipette seal in foil pouch together with test device
3. Operating Instruction
MATERIALS REQUIRED BUT NOT PROVIDED
1. Clock or Timer
2. A container for specimen collection
WARNING AND PRECAUTIONS
1. For in vitro diagnostic use only.
2. Do not use kit beyond the expiration date.
3. Urine specimens may be infectious; properly handle and dispose of all used reaction devices in biohazard container.

STORAGE
The kits should be stored at temperature 4-30°C the sealed pouch for
the duration of the shelf life (18 months).

ASSAY PROCEDURE
1. Bring the urine sample and test components to room temperature if refrigerated.
2. When ready to test, open the pouch at the notch and remove the test device. Place the test device on a clean, flat surface.
3. Fill the urine dropper with specimen. Holding the dropper vertically, dispense 2-3 drops (about 70-100µl) of urine without air bubble into the sample well.
4. Read the result in 5-10 minutes.
INTERPRETATION OF RESULTS

Negative: Two pink color bands appear. This indicates MOR in the specimen is below the detection sensitivity (300ng/ml).
Positive: Only one pink band for control appears. This indicates the MOR in the specimen is above the detection sensitivity.
Invalid: The presence of a control line is necessary to validate test performance. If no band appears the test should be repeated with a new test device.
Note: There is no meaning attributed to color intensity for C and T lines.
Limitation of The Procedure
1. This product is designed for use with human urine only
2. Although the test is very accurate, there is a possibility false results will occur due to the presence of interfering substances in the urine.
3. The test is a qualitative screening assay and is not for determining quantitative concentration levels or the level of intoxication.
4. Adulterants such as bleach or other strong oxidizing agents, when added to urine specimens may produce erroneous test results regardless of the analysis method used. If an adulteration is suspected, obtain another fresh urine specimen and retest.



 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!