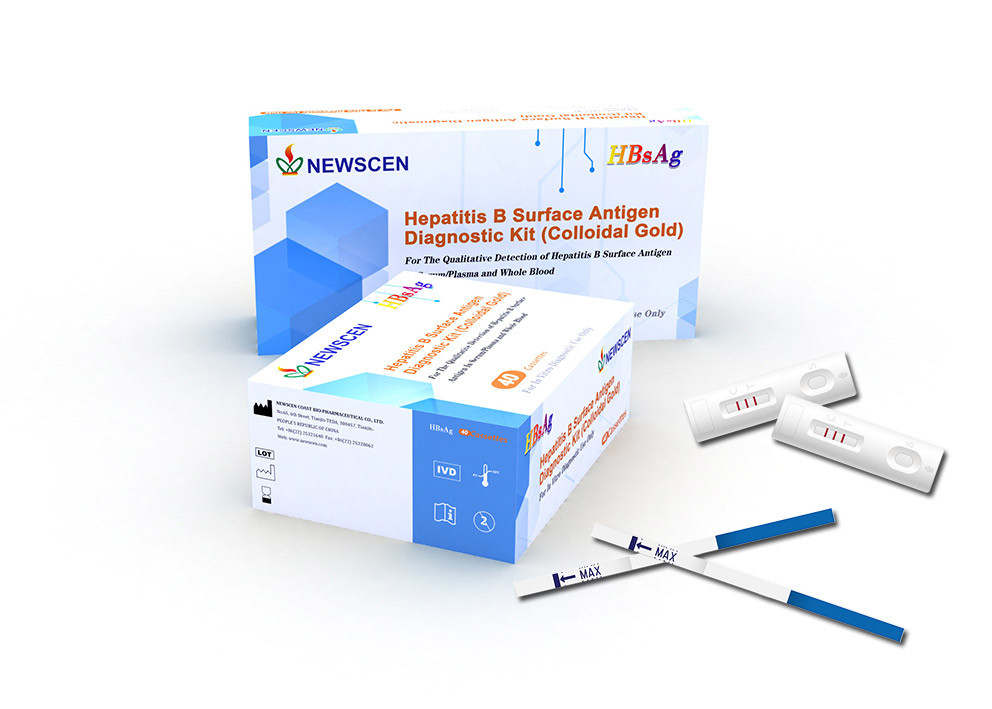
HBsAg (Hepatitis B Surface Antigen) Diagnostic Kit (Colloidal Gold)
For The Qualitative Detection of Hepatitis B Surface Antigen In Serum/Plasma and Whole Blood
Main Features
* High Sensitivity: > 96%
* Specificity: 100%
* Reliable: Accuracy higher than 98%, early detection of the presence of HBsAg Antigen.
* Simple: No Instrument Required
* Convenient: Room Temperature Storage Built-In Control
* Fast: Results in 5-30 minutes, strong positive results may be observed promptly within 3 minutes
* Certified by Authoritative Certification System and Standards
* Winner of "the National Rapid Diagnostic Kit for Clinical Performance Assessment"
Intended Use
The HBsAg Rapid Test is a Chromatographic immunoassay (CIA) for direct qualitative detection of Hepatitis B type virus surface antigen (HBsAg) in human serum /plasma and whole blood.

Principle
The HBsAg RDT is a chromatographic immunoassay (CIA) for the detection of surface antigens of Hepatitis B in human serum/plasma and whole blood. Specific antibody against HBsAg is pre-coated onto membrane as a capture reagent on the test region. During the test, specimen is allowed to react with the colloidal gold particles, which have been labeled with anotherr specific antibody. If HBsAg is present, a pink colored band will develop on the membrane in proportion to the amount of HBsAg presented in the specimen. Absence of this pink colored band in the test region suggests a negative result. To serve as a procedural control, a pink colored band in the control region will always appear regardless the presence or absence of HBsAg.

Storage
Store the test kits at temperature 4-30°C,in the sealed pouch for the duration of the shelf life (24 months).
Assay Procedure
Serum/Plasma
Add 70-100ul or 2-3 drops of serum or plasma into sample well. Observe the result in 5-20 minutes.
Whole blood
Add 1 drop of whole blood into sample well, after all blood is completely absorbed. Add 1 drop of whole blood diluent. Observe the result in 5-20 minutes.
Interpretation of Results

Negative:
No apparent band in the test region (T), a pink-colored band appears in the control region (C). This indicates that no HBsAg antibody has been detected.
Positive:
In addition to a pink-colored band in the control region (C), a pink-colored band will appear in the test region (T). This indicates that the specimen contains HBsAg.
Invalid:
If no band appears in the control region (C), regardless of the presence or absence of line in the test region (T). It indicates a possible error in performing the test. The test should be repeated using a new device.



 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!